Indication
PURIXAN is indicated for the treatment of patients with acute lymphoblastic leukemia (ALL) as a component of a combination maintenance therapy regimen.
Liquid Formulation
PURIXAN offers patients and healthcare professionals a liquid formulation as an alternative to the mercaptopurine tablet for precise dosing based on the individual patient’s dosing needs.
- Mercaptopurine is a cornerstone of all maintenance therapy drug regimens for acute lymphoblastic leukemia (ALL).1
- Until recently, the only marketed formulation of mercaptopurine has been a 50-mg tablet.
- However, solid oral dosage forms may be ineffective for many children because they have difficulty swallowing tablets and capsules.2
- Fixed doses are impractical because the dose varies according to the size of the child.2
- More than 90% of pediatricians reported that a drug’s taste and palatability were the greatest barriers to compliant treatment.2
- For children, PURIXAN addresses common concerns relating to treatment with a tablet.
Comparison between PURIXAN and the tablet form of mercaptopurine
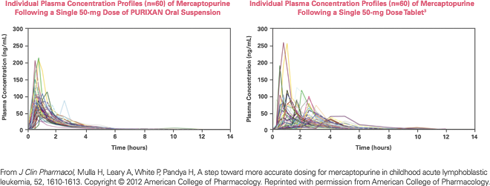
The bioavailability of PURIXAN is equivalent to that of the tablet form of mercaptopurine, as measured by the AUC (area under the concentration curve).
- The mean ratio of the AUCs for PURIXAN and mercaptopurine tablets is 114%, with a 90% confidence interval of 108%–121%. 3
- The accepted target range of AUC ratios for equivalence is 80%–125%.3
Predictability
PURIXAN performs more consistently and predictably than the mercaptopurine tablet.
- PURIXAN had substantially lower between-subject variability in maximum plasma concentrations (46% vs 69% coefficient of variation).3
Consistent Absorption
PURIXAN reduced the variability in the absorption of mercaptopurine, proving itself to be a dependable and reliable alternative to the tablet.3
Flexibility and Accuracy
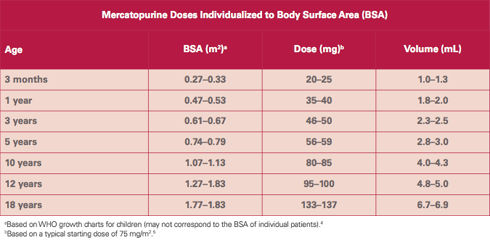
PURIXAN provides dosing flexibility and accuracy for precise dosing.
PURIXAN 20 mg/mL oral suspension provides an accuracy of 2 mg and an opportunity to administer the product in a way that may be more acceptable to children.2
- Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166-178.
- Mennella JA, Spector AC, Reed DR, Coldwell SE. The bad taste of medicines: overview of basic research on bitter taste. Clin There. 2013;35:1225-1246.
- Mulla H, Leary A, White P, Pandya H. A step toward more accurate dosing for mercaptopurine in childhood acute lymphoblastic leukemia. J Clin Pharmacol. 2012;52:1610-1613.
- WHO growth chart for children. http://www.who.int/childgrowth/standards/en/. Accessed April 16, 2020.
- PURIXAN (mercaptopurine) oral suspension [package insert]. Franklin, TN: Rare Disease Therapeutics, Inc.; April 2020.